How To Solve For Delta S Universe
Delta S Universe Delta S System Delta S Surroundings is greater than 0. Divide this enthalpy change by the temperature and you will get Delta S.
ΔSsurroundings ΔSsystemΔSsurroundings Δ S s u r r o u n d i n g s Δ S s y s t e m Δ S s u r r o u n d i n g.

How to solve for delta s universe. The universe tends toward disorder or randomness. Relate Delta S Universe for spontaneous processes and for processes at equilibrium. Thank you for the feedback.
DS rxn -262JK. Expert Answer 100 2 ratings Previous question Next question. Unlike the previous two examples the temperature has no affect on the spontaneous nature of the reaction.
How is it possible for a system to decrease its entropy. C DH rxn -135kJ. So the total entropy change of the Universe ie system surroundings brought about by the reaction is 307 J K 1 mol-1.
DeltaH -1760 kJ DeltaS -2848JK Solution. The enthalpy of the reaction is calculated to be -5384 kJ and the entropy of the reaction is 1017 JK. Remember this is why Delta G is useful for determining if reactions are spontaneous at constant T and P.
Show transcribed image text. Delta G system Delta H system - T Delta S system Delta G system - Delta H surroundings -. S surroundings H T 176 000298.
Delta S 1 mole1975 Jmole-K 1 mole223 Jmole-K - 1 mole2837 Jmole-K Delta S 4205 JK - 2837 JK 1368 JK Convert Delta S from JK to kJK. Energy transfer is necessary. The sign of ΔH can be or - but were just talking broadly and generally so lets start simply with ΔH However from the viewpoint of the surroundings the sign of ΔH changes when thermal energy is transferred from the system and becomes.
Discussion for teachers of AP Chemistry and help for students of AP Chemistry. Delta S cannot be less than 0. A DeltaH rxn -135kJ.
Please add any additional comments below. S surroundings 591 J K-1 mol-1 more than enough to outweigh the value of S system of 284 J K 1 mol-1. 24k members in the apchemistry community.
Delta S Universe Delta S System Delta S Surroundings 0 Use the thermodynamic table to determine Delta S reaction. With given reaction and thermodynamics quantities you can calculate delta S easily. In equation form we can write this as ΔS tot ΔS syst ΔS envir 0.
The total entropy change can be calculated by using the relation. The following is a list of things that increase or decrease entropy. In a irreversible process the total entropy of a system plus its surrounding increase.
DS rxn -262JK. How is delta S of the universe related to delta g of the system. Zns2HClg - ZnCl2sH2g Zns delta S is.
The change in enthalpy. Make sure you write units along the calculationWhat you need to be awar. For a spontaneous process.
Thus ΔS syst can be negative as long as ΔS envir is positive and greater in magnitude. Calculate DeltaS universe for the following reactions and predict if spontaneous. Did you find this video helpful.
Solution Only after calculating the enthalpy and entropy of the reaction is it possible for one can answer the question. DS rxn 262JK. Delta S univ 0.
How Is Delta S Of The Universe Related To Delta G Of The System. Calculate DeltaG from the formula DeltaG DeltaH - TDeltaS but first we need to convert the units for DeltaS into kJK or convert DeltaH into J and temperature into Kelvin. This problem has been solved.
For a equilibrium process. Delta S universe and Gibbs Free Energy. Solve for Delta G Delta S_textuniverse and Delta A Im also given latent heat of fusion Stack Exchange Network Stack Exchange network consists of 177 QA communities including Stack Overflow the largest most trusted online community for developers to.
B DH rxn 135kJ.
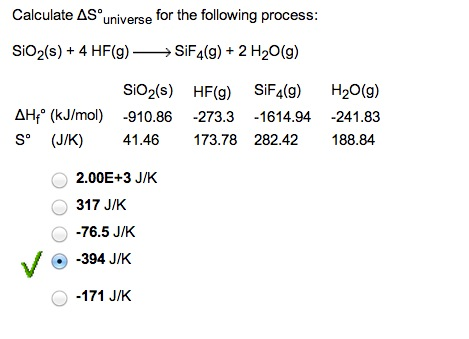
Calculate Delta S Degree Universe For The Following Chegg Com

Is It A Spontaneous Reaction Delta G Tells You Youtube

Answer Consider The Following Reaction At Clutch Prep

Quantum Principles Instructor Professor A J Shaka This Course Provides An Introduction To Qu Introduction To Quantum Mechanics Chemistry Quantum Mechanics

Calculate The Enthalpy Change Of Freezing Of 1 0 Mol Of Water At 10 C To Ice At 10 C Youtube

Answer Consider The Following Reaction At Clutch Prep

M1 Seminar Week 1 Introduction To Nonlinear Wave Equations Math Mathematics Osaka Osakauniversity Pde Wave Equation Equations Mathematics

How To Calculate Entropy Changes Liquids Solids And Phase Changes Youtube

Calculate The Standard Entropy D S Rxn O Clutch Prep

Calculate The Standard Entropy D S Rxn O Clutch Prep

Calculating Molar Solubility With No Ksp Solubility Chemistry Worksheets High School Chemistry

Gibbs Free Energy Ciencias Biblia

The Equation Of Motion Newton S Second Law In A Rotating Frame Navier Stokes Physics And Mathematics Equations Math Notation

About The Chemical Reaction The Chemical Reaction In Rocket Fuel Chemical Reactions Balancing Equations Chemical

Calculate The Entropy Change In The Surroundings Youtube

Thermodynamics 9 4 The Entropy Change Of The Universe Youtube



